
Emerging Technologies for the In Situ
Remediation of PCB-Contaminated
Soils and Sediments:
Bioremediation and Nanoscale
Zero-Valent Iron
August 2004
Prepared by
Alex Mikszewski
National Network for Environmental Management Studies Fellow
for
U.S. Environmental Protection Agency
Office of Solid Waste and Emergency Response
Office of Superfund Remediation and Technology Innovation
Technology Innovation Program
Washington, DC
www.clu-in.org

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Notice
This document was prepared by a student participating in the Cornell University Internship
Program for the U.S. Environmental Protection Agency (EPA). This report was not subject to
EPA peer review or technical review. The EPA makes no warranties, expressed or implied,
including, without limitation, warranties for completeness, accuracy, usefulness of the
information, merchantability, or fitness for a particular purpose. Moreover, the listing of any
technology, corporation, company, person, or facility in this report does not constitute
endorsement, approval, or recommendation by EPA.
The report contains information gathered from a range of currently available sources, including
project documents, reports, periodicals, Internet searches, and personal communication with
involved parties. No attempts were made to independently confirm the resources used. It has
been reproduced to help provide federal agencies, states, consulting engineering firms, private
industries, and technology developers with information on the current status of this project.
This report reviews emerging technologies for the in situ remediation of PCB-contaminated
sediments and soils to assess their viability for future employment. The target audience is federal
and state regulators, planners, and managers responsible for cleaning up soils and sediments
contaminated with PCBs. The report is available on the Internet at
www.clu-in.org/
studentpapers/.
The author would like to thank everyone in EPA’s Technology Innovation and Field Services
Division for their support and advice. Special thanks are extended to Linda Fiedler, Jon Josephs,
Kelly Madalinski and Greg Wilson. The author also wishes to thank Dr. Ruth Richardson, Dr.
James Tiedje, Dr. Greg Lowry, Dr. Kevin Gardner, Dr. Simon Jackman, and Dr. John Tharakan
for their expertise.
About the National Network for Environmental Management Studies (NNEMS)
NNEMS is a comprehensive fellowship program managed by the EPA’s Office of Environmental
Education. The purpose of the NNEMS Program is to provide students with practical research
opportunities and experiences.
Each participating headquarters or regional office develops and sponsors projects for student
research. The projects are narrow in scope to allow the student to complete the research by
working full-time during the summer or part-time during the school year. Research fellowships
are available in Environmental Policy, Regulations, and Law; Environmental Management and
Administration; Environmental Science; Public Relations and Communications; and Computer
Programming and Development.
NNEMS fellow receive a stipend at a level determined by the student’s level of education, the
duration of the research project, and the location of the research project. Fellowships are offered
to undergraduate and graduate students. Students must meet certain eligibility criteria.
i

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Contents
Page
I. Purpose ...................................................................1
II. Characterization of the Problem ................................................1
III. Public Health Implications ...................................................2
IV. Traditional Remediation Technologies ..........................................3
V. Anaerobic Reductive Dechlorination ............................................3
Background ...............................................................3
Analysis of Dechlorinating Populations .........................................5
The Identification of Two Dechlorinating Organisms ...............................5
Organism o-17 ..........................................................5
Organism DF-1 .........................................................6
Biostimulation .............................................................6
Bioaugmentation ...........................................................7
Technology Assessment ......................................................7
Analysis of a Summer 2003 Field Study .........................................8
VI. Aerobic Biodegradation .....................................................9
Background ...............................................................9
Analysis of PCB-Degrading Populations and Mechanisms ...........................9
Genetic Engineering for PCB Mineralization: Strain RHA(pRHD34) .................10
Problems in the Pathway ....................................................11
A Superior Recombinant Strain: LB400(pR041) ..................................12
Remaining Barriers and Possible Remedies .....................................12
Revisiting the 1991 GE Hudson River Field Study ................................13
VII. Reductive Dechlorination by Nanoscale Zero-Valent Iron .........................13
Background ..............................................................13
Demonstrating the Potential of Nanoscale ZVI ...................................14
Analysis of ZVI Positional Preferences .........................................14
PCB Dechlorination by Micro- and Nanoscale ZVI in Contaminated Sediments .........15
Conflicting Research .......................................................16
Improving ZVI Longevity ...................................................16
Synergistic Dechlorination by ZVI and Anaerobic Organisms .......................16
Enantiomeric and Isotopic Fractionation ........................................17
Technology Assessment .....................................................18
ii

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
VIII. The “Availability” Problem .................................................18
IX. Conclusion ..............................................................19
X. Citations .................................................................20
Figures
Figure 1. Biphenyl Molecule.....................................................1
Figure 2. Microbial Dechlorination Pathways .......................................4
Figure 3. Biphenyl (bph) Pathway ................................................9
Figure 4. DHBD Cleavage .....................................................11
iii
Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
I. Purpose
Persistent organic pollutants foul countless aquatic ecosystems worldwide. The remediation of
these contaminants is essential to promote public health, environmental quality, and the
economy. Polychlorinated biphenyls (PCBs) reside in river sediments for extended durations and
bioaccumulate in the food chain through predation (Bedard, 2003). Traditional remediation
practices for these contaminants have serious limitations and high costs. The mission of this
document is to review emerging technologies for in situ remediation of PCB-contaminated
sediments and soils and to assess their viability for future employment. Emphasis is placed on
bioremediation and the use of nano-sized zero-valent iron for reductive dechlorination.
II. Characterization of the Problem
PCBs are synthetic aromatic compounds notorious for their recalcitrance and potential toxicity.
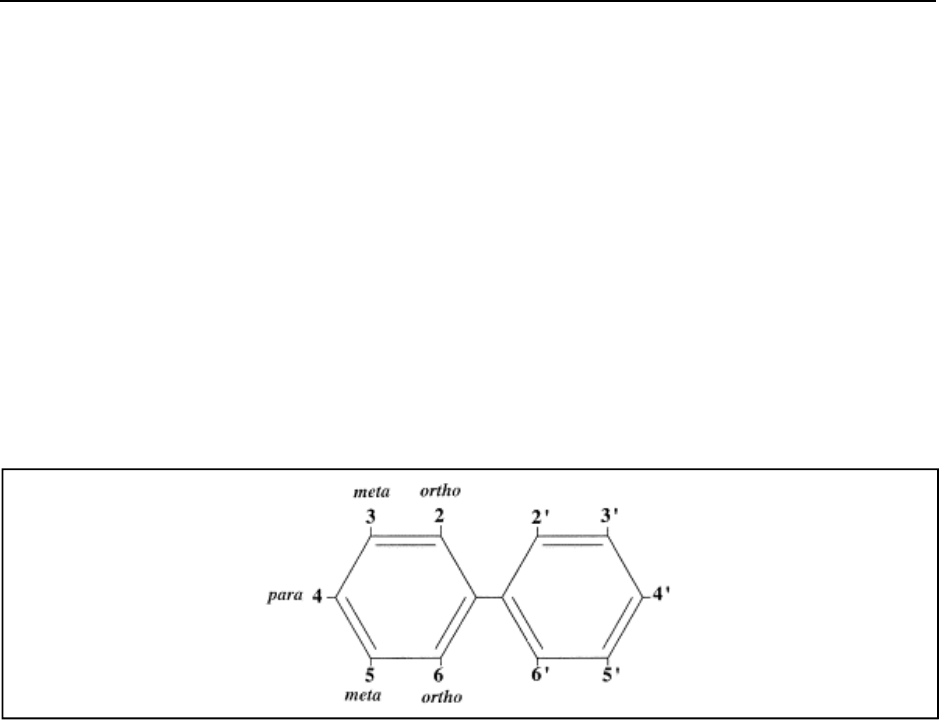
PCBs comprise two benzene rings connected at the C-1 carbon (Wiegel and Wu, 2000). Each
benzene ring can have up to 5 chlorine substituents in the ortho, meta, or para positions (See
Figure 1. Biphenyl Molecule
(Wiegel and Wu, 2000)
figure 1) (Wiegel and Wu, 2000). PCBs thus have 209 distinct structural arrangements differing
in chlorine number and position (Bedard, 2003). Each species is known as a congener and
exhibits unique chemical properties (Bedard and May, 1996). In the United States, PCBs were
sold commercially as mixtures, most commonly under the trade name Aroclor (Wiegel and Wu,
2000).
American industries manufactured PCBs from 1929 to 1978 primarily for use in electrical
transformers and capacitors (Bedard, 2003). PCBs are wonderful insulators characterized by their
stability, incombustability, and low volatility (Rodrigues et al., 2000). PCB production in the
United States peaked in 1975, as their indestructability made them suitable for a myriad of
industrial purposes (Abraham et al., 2002). The widespread use of PCBs inevitably resulted in
their deliberate and unintentional discharges into the environment. One-third of all U.S.-
produced PCBs currently reside in the natural environment (Wiegel and Wu, 2000).
1

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Once in aquatic or terrestrial systems, PCBs sorb to abiotic or biotic particles due to their
hydrophobicity (Mondello, 2002). Heavily chlorinated congeners are the most water insoluble
(Mondello, 2002). Of the hundreds of millions of pounds of PCBs released into the environment,
most are bound to aquatic sediments (Bedard, 2003). PCBs are recalcitrant to biological
degradation because they are so highly oxidized (Mondello, 2002). Furthermore, strongly sorbed
PCB molecules are not available to microorganisms capable of PCB degradation. Deposition of
clean sediments slowly buries PCB-contaminated particles, reducing the risk of human exposure;
however, elevated flows can resuspend contaminated sediments, making PCBs available to
aquatic organisms once again (QEA, 1999). The slow desorption of PCBs also pollutes the water
column, making the natural recovery of contaminated sediments an ineffective remediation
mechanism. PCBs were banned in the United States in 1978 due to growing concern about their
toxicity and environmental longevity (Wiegel and Wu, 2000).
III. Public Health Implications
PCBs pose a very real human health threat through numerous exposure pathways. Most alarming
is the tendency of PCBs to bioaccumulate, or to increase in concentration while ascending the
food chain. PCB concentrations in fish and aquatic mammals can be thousands of times higher
than levels in the surrounding waters (Rahuman et al., 2000). Contaminated fish consumption is
a major route of PCB bioaccumulation in humans (Johnson et al., 2000). Other exposure avenues
are usage of old electrical appliances and inhalation of volatilized PCBs near contaminated sites
(Rahuman et al., 2000). Laboratory animals dosed with PCBs developed numerous health
problems. Among the adverse health effects were liver damage, skin irritation (acne),
reproductive dysfunction, and cancer (Rahuman et al., 2000). Humans exposed to PCBs have an
increased risk of developing cancers like non-Hodgkins lymphoma (Johnson et al., 2000).
Research also has shown that PCBs can cause severe neurological problems in children,
including impairment of cognitive and motor abilities (Faroon et al., 2001). Lipophilic PCBs can
be transmitted from mother to child during breast feeding (Faroon et al., 2001).
PCBs are considered most dangerous in their potential for a “dioxin-like toxicity” (Baars et al.,
2004). Dioxins are organic aromatic compounds released by industrial processes, seismic
emissions, or waste incineration emissions (Baars et al., 2004). They can be chlorinated and are
regarded as much more toxic than PCBs. Dioxins cause immunological and reproductive
dysfunction and inhibit neurologic growth and development (Baars et al., 2004). The U.S.
Environmental Protection Agency regulates dioxins as probable carcinogens, and 2,3,7,8-
tetrachloro-dibenzo-p-dioxin is considered the most toxic synthetic chemical ever produced
(Gruden et al., 2003; Halden and Dwyer, 1997). Dioxin-like PCB congeners contain two
chlorines in the para position, at least two chlorines in the meta position, and at most one
chlorine in the ortho position (Bedard, 2003). This arrangement allows the PCB molecule to
rotate and assume a coplanar orientation, causing the dioxin-like behavior (Baars et al., 2004).
While dioxin-like PCBs are more carcinogenic, non-coplanar congeners are more disruptive of
2

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
cognitive function (Faroon et al., 2001). To protect public health, all congeners of PCBs must be
completely removed from polluted sites available to human exposure.
IV. Traditional Remediation Technologies
Incineration and landfilling are two traditional methods for remediation of PCB-contaminated
soils and sediments. High temperature incineration is most commonly used for complete
destruction of PCBs (Rahuman et al., 2000). Specialized incinerators burn PCB-contaminated
soils or sediments at temperatures up to 1200
B
C and are required to achieve removal efficiencies
of 99.999 percent (U.S. EPA, 1997). There is much public opposition to hazardous waste
incineration for fear of exposure to toxic emissions. Furthermore, incineration is very expensive,
costing up to $2,300 per ton for a fixed PCB incinerator (U.S. EPA, 1997).
Sequestering liquid PCBs or contaminated soils and sediments in a hazardous waste landfill is
another form of disposal (U.S. EPA, 1997). The main associated danger is that the PCBs can
volatilize and escape the landfill through surrounding air channels (Rahuman et al., 2000). The
failure of leachate collection systems also could result in PCB groundwater infiltration.
Landfilling is merely a containment mechanism that does not eliminate the possibility of
environmental contamination. The crippling limitation of landfilling and incineration is that they
can only be applied ex situ. As a result, dredging of river sediments and soil excavation are
necessary precursors to PCB destruction. Aquatic PCB contamination can be temporarily
worsened by the dredging process.
Dredging stirs up a fraction of the PCBs formerly tied to sediments, resuspending them in the
water (Voie et al., 2002). Dredging also removes organic fine grained sediments, leaving behind
coarse inorganics with a lower affinity for PCB binding. As a result, PCBs become temporarily
more concentrated in the water column, and consequentially more available for bioaccumulation
in aquatic wildlife (Voie et al., 2002). Like incineration, dredging is an expensive procedure. The
proposed Superfund dredging of contaminated sediments in the Hudson River will cost upwards
of half a billion dollars (U.S. EPA, 2003). Although dredging is proven to be effective in the long
run, a more efficient in situ strategy would facilitate the remediation of contaminated soils and
sediments.
V. Anaerobic Reductive Dechlorination
Background
In the 1980s, researchers noted discrepancies between commercial Aroclor mixtures and PCBs
found in contaminated sediments. The congener distribution of sediment PCBs had a greater
proportion of lightly chlorinated species (Wiegel and Wu, 2000). The apparent dechlorination
processes occurring naturally in contaminated sediments stimulated extensive laboratory work, as
it was once thought that chlorinated synthetic compounds were completely resistant to microbial
breakdown (Mondello, 2002). Research soon conclusively demonstrated that anaerobic
3
Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
organisms were responsible for the PCB dechlorination in aquatic sediments (Bedard, 2003).
Anaerobic bacteria replace chlorine substituents with the electron-donating hydrogen (from H
2
)
on the PCB molecule (Wiegel and Wu, 2000).
In general, microbial reductive dechlorination of PCBs removes meta and para chlorines from
highly chlorinated congeners, resulting in predominately ortho substituted mono- through
tetrachlorobiphenyls (Wiegel and Wu, 2000). There are eight major dechlorination pathways
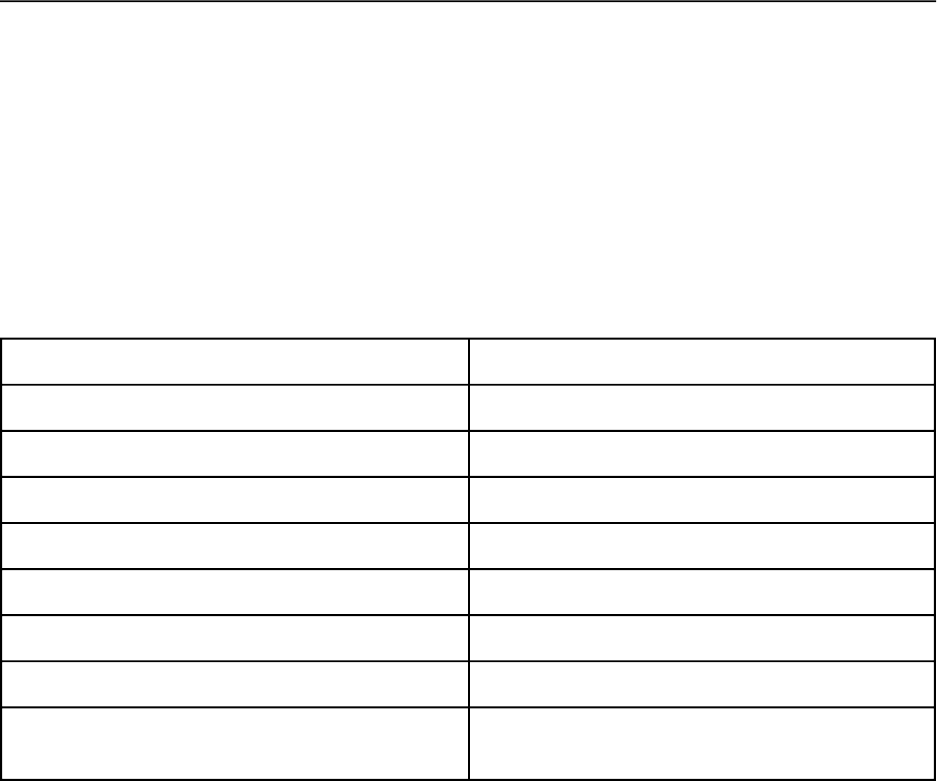
known to date, each differing in congener and position reactivity (Bedard, 2003). Figure 2
reviews the known microbial dechlorination processes. The most extensive dechlorination occurs
when process M works in combination with process Q. This activity, known as C
Dechlorination Pathway Chlorines Removed
M Flanked and unflanked meta
Q Flanked and unflanked para, meta of 2,3-
H’ Flanked para, meta of 2,3- and 2,3,4-
H Flanked para, doubly flanked meta
P Flanked para
N Flanked meta
LP Flanked and unflanked para
T Flanked meta of 2,3,4,5- in hepta- and
octachlorobiphenyls
Figure 2. Microbial Dechlorination Pathways
“Flanked” signifies an adjacent chlorine
(Wiegel and Wu, 2000)
dechlorination, voraciously attacks meta and para chlorines, resulting in exclusively ortho
substituted congeners (Zwiernik et al., 1998). This process is advantageous because lightly
chlorinated ortho substituted species are non-dioxin-like and do not readily bioaccumulate.
Unfortunately, only Hudson River sediments have expressed process C dechlorination in situ
(Zwiernik et al., 1998). While no defined ortho dechlorination pathways exist, enriched cultures
derived from Baltimore Harbor estuarine sediments exhibit significant ortho dechlorination
(Berkaw et al., 1996).
4

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Analysis of Dechlorinating Populations
Characterization of the anaerobic organisms responsible for PCB dechlorination is paramount to
the development of a remedial scheme. PCB reduction is known to occur as a cometabolic
process and is believed to occur as a product of dehalorespiration (Abraham et al., 2002).
Methanogens and sulfate reducers are largely responsible for dechlorination pathways H, M, and
Q (Bedard, 2003). Spore-forming sulfate reducers are the most important, essential for the para
dechlorination in process Q (Zwiernik et al., 1998). Furthermore, Fava reports that spore-forming
sulfate reducers are necessary for M dechlorination (Fava et al., 2003a). M dechlorination does
not proceed in the presence of molybdate, an inhibitor of sulfate-reducing bacteria (Fava et al.,
2003a). Sulfate-reducing bacteria are thus responsible for C dechlorination, the most prolific
dechlorination process found in contaminated sediments.
Dehalorespiration refers to microbial use of halogenated compounds as terminal electron
acceptors for energy synthesis (Rosenthal et al., 2004). The identification of a PCB-respiring
organism would be invaluable to the advancement of PCB bioremediation. Such a microbe could
use PCB for growth, thus having a distinct advantage in PCB-contaminated sediments. Kim and
Rhee demonstrated that such dechlorinating organisms exist in sediments independent of sulfate
reducers or methanogens (Kim and Rhee, 1997). The study showed that components of an
anaerobic consortium required Aroclor 1248 for growth, disappearing below a threshold
concentration (Kim and Rhee, 1997). The populations of sulfate reducers and methanogens were
sustained in the absence of the Aroclor (Kim and Rhee, 1997).
The Identification of Two Dechlorinating Organisms
Organism o-17
In the past few years, two different individual species have been identified that catalyze the
reductive dechlorination of PCBs. The first such microbe is bacterium o-17, which depends on
the presence of 2,3,5,6-tetrachlorobiphenyl for growth (Cutter et al., 2001). Cutter derived o-17
from the ortho dechlorinating consortium of Baltimore Harbor. In sediment-free media, o-17
reduces congeners 2,3,5,6- and 2,3,5-chlorobiphenyl to 3,5-chlorobiphenyl. Acetate is a potential
electron donor for the process, as o-17 requires acetate for dechlorination (Cutter et al., 2001).
Addition of hydrogen to the medium inhibited dechlorination, suggesting that hydrogen is not the
main electron donor. Yet hydrogen produced from the oxidation of acetate might serve as the
donor, so the oxidation-reduction mechanism remains unclear (Cutter et al., 2001). Attempts to
isolate o-17 as a pure culture have been unsuccessful (Cutter et al., 2001).
As the ortho position is notoriously resistant to reductive dechlorination, the discovery of o-17
constitutes a major breakthrough. Phylogenetically, o-17 is most similar to Dehalococcoides
ethanogenes, a hydrogenotrophic organism that respires through the dechlorination of tetra-
chloroethene (Cutter et al., 2001). The two organisms and the chlorobenzene dechlorinating
strain Dehalococcoides CDB1 belong to a phylogenetic branch closely related to the green non-
5

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
sulfur bacteria (Cutter et al., 2001). The fact that o-17 strongly resembles the only known
organisms capable of dehalorespiration is very encouraging (Abraham et al., 2002).
Unfortunately, the estuarine origin of o-17 limits its compatibility with the environmental
conditions of soils and sediments. Such site-specific dechlorination activity is unlikely to evolve
into a ubiquitous remedial solution.
Organism DF-1
Wu discovered another organism with growth linked to the reductive dechlorination of PCBs
(Wu et al., 2002). Bacterium DF-1 dechlorinates doubly flanked chlorines on the biphenyl
molecule (Wu et al., 2002). DF-1 can remove meta chlorines from 2,3,4-chlorobiphenyl and
2,3,4,6-chlorobiphenyl, and para chlorines from 3,4,5-chlorobiphenyl and 2,3,4,5-chlorobiphenyl
(Wu et al., 2002). DF-1 was identified as the responsible dechlorinator from a culture containing
mainly sulfate reducers; thus, the extensive dechlorination capacity of sulfate-reducing consortia
might be attributable to specific dechlorinating bacteria, such as DF-1. The bacterium most
closely resembles o-17 (89 percent rDNA sequence similarity), further advancing the thought that
a class of PCB dechlorinators exists in the natural environment (Wu et al., 2002). DF-1, like o-
17, has yet to be isolated as a pure culture (Wu et al., 2002).
Biostimulation
The two major bioremedial actions are biostimulation and bioaugmentation. Biostimulation
involves the addition of a primer to galvanize targeted dechlorinating populations. A very
successful laboratory study stimulated process C dechlorination through the addition of ferrous
sulfate to PCB-contaminated soils (Zwiernik et al., 1998). FeSO
4
amendments saturate aqueous
systems with free sulfate, which is consumed by sulfate reducers. Bioenergetics favor the sulfate
reducers over the methanogens, and sulfate-reducing populations grow rapidly, while
methanogenic growth is inhibited (Zwiernik et al., 1998). PCB dechlorination is initially
inhibited as sulfate becomes the primary electron acceptor for microbial respiration. Once sulfate
is depleted, PCB dechlorination resumes as the sulfate reducers attack para chlorines,
supplementing the more common meta dechlorination observed in the unamended controls
(Zwiernik et al., 1998). The result is nearly complete C dechlorination of Aroclor 1242, resulting
in the accumulation of the ortho substituted congeners 2-chlorobiphenyl and 2,2'/2,6-chloro-
biphenyl (Zwiernik et al., 1998). This process has great potential for in situ application, as it was
postulated that priming one ton of sediment requires only 10.6 pound of ferrous sulfate, a cheap
and environmentally benign product (Bedard, 2003).
Another way to “prime” anaerobic sediments for PCB dechlorination is through addition of
bromobiphenyls. A field study in Woods Pond of the Housatonic River demonstrated that spiking
sediments with 2,6-bromobiphenyl stimulated reductive dechlorination (Bedard, 2003). One 350
:M pulse of the bromobiphenyl activated native PCB dechlorinators, resulting in a 74 percent
decrease in PCBs with six or more chlorines in just one year (Bedard, 2003). The bromobiphenyl
primer resulted in N dechlorination, yielding mainly ortho and para substituted tetrachlorobi-
6

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
phenyls (Bedard, 2003). Inoculation of sediments with N dechlorination products can prime
sediments for LP dechlorination, resulting in mostly ortho substituted dichlorobiphenyls (Bedard,
2003). The downside of using halogenated aromatics as primers is that they are recalcitrant to
degradation (Abraham et al., 2002). The practice of adding more of a contaminant to a site to
stimulate microbial action is unacceptable to regulators. Halobenzoates are easily mineralized
and are thus more suitable as dechlorination primers. Chlorobenzoates result from the aerobic
oxidation of lightly chlorinated PCBs. Interestingly, they successfully stimulate dechlorination
only in sediments other than their sediments of origin (Abraham et al., 2002). Priming is
necessary to incite and expedite the reductive dechlorination of PCBs.
Bioaugmentation
Bioaugmentation is the process of enriching a contaminated site with organisms capable of
degrading a targeted compound. Attempts to augment PCB-dechlorinating cultures in Housatonic
River sediments have been unsuccessful (Bedard, 2003). These studies inoculated the sediments
with enriched cultures indigenous to the Housatonic River (Bedard, 2003). Augmentation with
cultures from different PCB-contaminated sediments might have worked better, just as chloro-
benzoates only prime dechlorination in non-native sediments. One successful augmentation study
used a granular anaerobic methanogenic microbial consortium (Natarajan et al., 1996). The
granules were produced by an upflow anaerobic sludge-blanket reactor with a continuous supply
of carbon and electron sources (Natarajan et al., 1996). In the laboratory, the methanogenic
granules completely dechlorinated 2,3,4,5,6-pentachlorobiphenyl to biphenyl. Dechlorination to
biphenyl was an unprecedented accomplishment. The granules removed chlorines from all
feasible positions in the presence of glucose and methanol (Natarajan et al., 1996).
In a subsequent study, the granular consortium was added to PCB-contaminated sediments from
the River Raisin (Natarajan et al., 1997). Sediment amended with the granules experienced a
significant reduction in tri- through heptachlorobiphenyls (Natarajan et al., 1997). The primary
dechlorination products of the original Aroclor 1242 and 1248 mixtures were ortho substituted
mono- and dichlorobiphenyls (Natarajan et al., 1997). Control sediments without the inoculum
underwent very slight dechlorination, illustrating the success of the bioaugmentation. Also
encouraging was that the granules dechlorinated at a wide range of ambient temperatures
(Natarajan et al., 1997). Potentially problematic is that the bench-scale experiments required a
volume of granules equal to 10 percent of the treated sediment volume (Bedard, 2003). Questions
remain as to how the efficiency of the consortium translates to full-scale field projects. The last
few years have shown no advances in the granular technology, which suggests that it is not as
promising as once imagined.
Technology Assessment
There is great potential for in situ remediation of PCB-contaminated sites using anaerobic
reductive dechlorination. Dechlorination pathways have been identified along with two
organisms that catalyze the reductive dechlorination of PCBs. DF-1 and o-17 are very similar to
7

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
each other and to Dehalococcoides ethenogenes, a bacteria known to halorespire on
tetrachloroethene (Wu et al., 2002). Even more intriguing is a recent study reporting that
Dehalococcoides (Dhc) ethenogenes strain 195 dechlorinates 2,3,4,5,6-pentachlorobiphenyl to
2,3,4,6- and/or 2,3,5,6-tetrachlorobiphenyl and 2,4,6-trichlorobiphenyl (Fennell et al., 2004).
Researchers did not test Dhc 195 for growth on PCB, but the strain was shown to use chlorinated
benzenes as electron acceptors (Fennell et al., 2004). This report supports the idea that certain
Dhc species are involved in the natural reductive dechlorination of PCBs. Research must
determine if Dhc strains are able to use PCB for growth.
At present, anaerobic reductive dechlorination is not a viable stand-alone alternative to
dredging/excavation and burning. More field studies must be conducted to test methods of
bioaugmentation and biostimulation. The behavior of PCB-dechlorinating enrichment cultures
has not been evaluated in situ. Methods of priming dechlorination are established, but their field
applicability is unknown. Pure culture isolation of a PCB dechlorinator is essential in developing
a better understanding of the relevant microbial processes (Wiegel and Wu, 2000). As described
below, a recent attempt at field-scale remediation of PCBs was largely unsuccessful, but it is
useful for illustrating the remaining barriers to biotic dechlorination of PCBs.
Analysis of a Summer 2003 Field Study
In the summer of 2003, the Army Corps of Engineers sponsored a field-scale bioremediation test
on PCB-contaminated soils in Mississippi (Tiedje, 2004). The project sought to mineralize a
mixture of Aroclors 1242/1248 through a sequential anaerobic/aerobic treatment (Tiedje, 2004).
Researchers from Michigan State devised the remediation scheme with the idea that anaerobic
reductive dechlorination would reduce chlorination to levels low enough for aerobic oxidation to
cleave the biphenyl molecule. Unfortunately, attempts to stimulate reductive dechlorination were
unsuccessful (Tiedje, 2004). Researchers applied PCB-contaminated sediment and a carbon
source to the flooded soil in an effort to trigger dechlorinators already present in the soil. After
six months, no substantial dechlorination was observed, and the Corps terminated the project
(Tiedje, 2004).
The project in Mississippi highlights the shortcomings of anaerobic reductive dechlorination as a
remedial process for PCB-contaminated soils and sediments. Significant dechlorination can take
several years under optimal environmental conditions (Tiedje, 2004). The six-month time limit
was highly unreasonable. Aside from time constraints, the limited bioavailability of PCBs
severely inhibits reductive dechlorination. PCBs are often tightly bound to soil and sediment
particles, rendering them resistant to the enzymes of dechlorinators (Richardson, 2004).
Furthermore, it is very difficult to establish and stimulate PCB-dechlorinating organisms in
remediation sites. Threshold PCB concentrations exist for the successful maintenance of
dechlorinating cultures that might not be abundant in the first place (Cho et al., 2003). The
interactions of the mechanisms involved must be studied further, along with the properties of the
PCB dechlorinators themselves.
8
Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
VI. Aerobic Biodegradation
Background
Preliminary laboratory research on aerobic PCB biodegradation was discouraging. Researchers
tried to identify organisms capable of utilizing highly chlorinated PCBs as carbon sources for
growth (Mondello, 2002). In 1973, Ahmed and Focht reported that Achromobacter degrades a
few lightly chlorinated PCBs as a cometabolic function of biphenyl oxidation (Mondello, 2002).
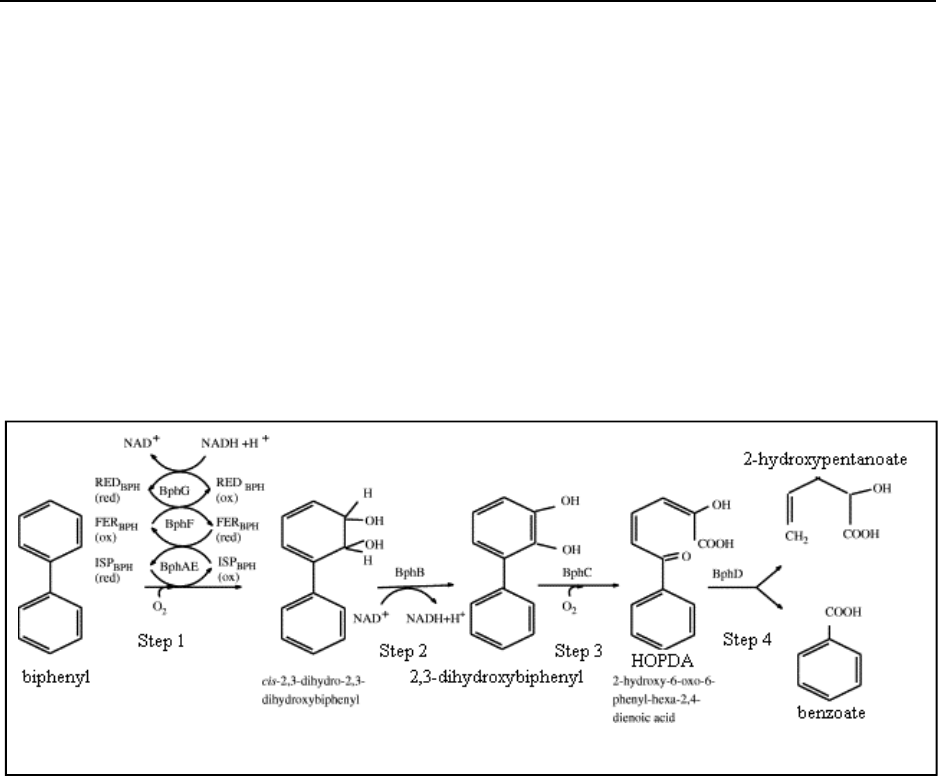
It is now well known that PCBs are broken down by the catabolic “biphenyl pathway” (or bph
pathway) (Sylvestre, 2004). The bph pathway is a four-step enzymatic process that turns biphenyl
into benzoic acid and 2-hydroxy-penta-2,4-dienoic acid (Bedard, 2003) (See figure 3 for bph
pathway schematic). The pentanoic acid product is effectively converted to acetyl-CoA and used
in the tricarboxylic acid cycle (Bedard, 2003). In general, PCBs with at most three chlorines are
susceptible to degradation via the bph pathway (Mondello, 2002).
Figure 3. Biphenyl (bph) Pathway
(Sylvestre, 2004)
Analysis of PCB-Degrading Populations and Mechanisms
A broad range of gram-negative and gram-positive aerobic bacteria encoding the biphenyl
pathway are capable of cometabolically degrading PCBs. The large majority use biphenyl
dioxygenase to attack 2,3- carbons and form 2,3-dihydrodiol (Mondello, 2002). Other
dioxygenases subsequently produce 2,3-dihydroxychlorobiphenyl, which is cleaved at the meta
position to yield chlorinated 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid (HOPDA)
(Sylvestre, 2004). This stage can be a “bottleneck” for certain PCB congeners because 3- and 4-
chloroHOPDA competitively inhibit HOPDA hydrolase (Bedard, 2003; Sylvestre, 2004). If
uninhibited, the hydrolase splits HOPDA into chlorobenzoic acid and a five-carbon compound
(Mondello, 2002).
9

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Bukholderia cepacia LB400 and Ralstonia eutropha H850 are distinguished by their broad
congener specificities, and thus have been the subjects of extensive research. LB400 is the most
capable PCB degrader and is not pathenogenic like its relative, Burkholderia pseudomallei
(Kenyon College, 2004). LB400 and H850 are unique because they can attack PCBs without
unchlorinated 2,3- positions (Mondello, 2002). The biphenyl oxygenase of LB400 and H850 has
an acute affinity for 2- and 2,4-chlorophenyl rings at the ortho position. As a result, they can use
oxygenolytic dehalogenation to spontaneously produce dihydroxybiphenyl (Bedard, 2003). A
number of competent gram-positive PCB degraders belong to the Rhodococcus genus (Mondello,
2002).
Genetic Engineering for PCB Mineralization: Strain RHA(pRHD34)
The mineralization of PCB by the biphenyl pathway is extremely rare. Most of the time, the
enzymes degrade the ring with fewer chlorines while releasing the second ring as a chlorobenzoic
acid (CBA) (Abraham et al., 2002). Natural PCB degraders are unable to catalyze the degradation
of chlorobenzoates, leading to a buildup of the metabolite (Rodrigues et al., 2000). This is
problematic because CBAs can be toxic and inhibitory to PCB degraders (Rodrigues et al.,
2000). As a result, genetic engineering has become a necessary tactic to produce organisms with
the bph pathway and a CBA degradation pathway. An organism capable of completely
mineralizing a wide range of congeners would advance bioremediation as a viable alternative for
PCB-contaminated sites.
Aerobic degradation of PCBs is severely limited by the inability of naturally occurring organisms
to grow on and fully metabolize the PCB molecule (Rodrigues et al., 2000). Efficient PCB
destruction by the bph pathway is dependent upon the availability of biphenyl as a co-substrate
(Manzano et al., 2003). A major development was the construction of a recombinant
Rhodococcus RHA1 strain capable of growing on PCB in non-sterile soil media (Rodrigues et
al., 2000). RHA1 degrades a wide range of PCBs and co-contaminants, like benzene (Bedard,
2003). Naturally occurring RHA1 does not use PCB as a carbon source, and cannot degrade the
chlorobenzoic acids that accumulate as a product of PCB cometabolism (Rodrigues et al., 2000).
Researchers first identified the fcb operon as the genes encoding for the hydrolytic dechlorination
of 4-CBA (Rodrigues et al., 2000). The operon was cloned into the RHA1 strain to supplement
the already present bph pathway. The resulting modified organism, RHA(pRHD34), was able to
grow on and degrade 4-chlorobiphenyl without subsequent accumulation of 4-CBA (Rodrigues et
al., 2000).
Wild-type RHA1 converts approximately 60 percent of process M dechlorination products to
corresponding CBAs (Rodrigues et al., 2000). The recombinant strain breaks down PCBs with
similar efficiency and completely degrades 4-CBA. RHA(pRHD34) also reduces meta cleavage
products that can inhibit enzymatic function (Rodrigues et al., 2000). The fcb operon was stable in
the non-sterile media for 60 days, which should be more then enough time for an aerobic field
remediation project (Rodrigues et al., 2000).
10
Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
RHA(pRHD34) shows great promise for remediation of PCBs but has many limitations. For one,
growth on 4-chlorobiphenyl is only feasible through partial induction of the bph pathway. Full
expression of the bph pathway produces 4-CBA faster than the fcb pathway can break it down
(Bedard, 2003). The result is accumulation of 4-chloroHOPDA that inhibits growth of the
recombinant strain (Rodrigues et al., 2000). Partial induction of bph ameliorates this problem but
limits the range of PCBs degraded by the recombinant strain (Bedard, 2003). It is essential,
therefore, to engineer organisms with the fcb operon that still allow full expression of bph
(Bedard, 2003).
Problems in the Pathway
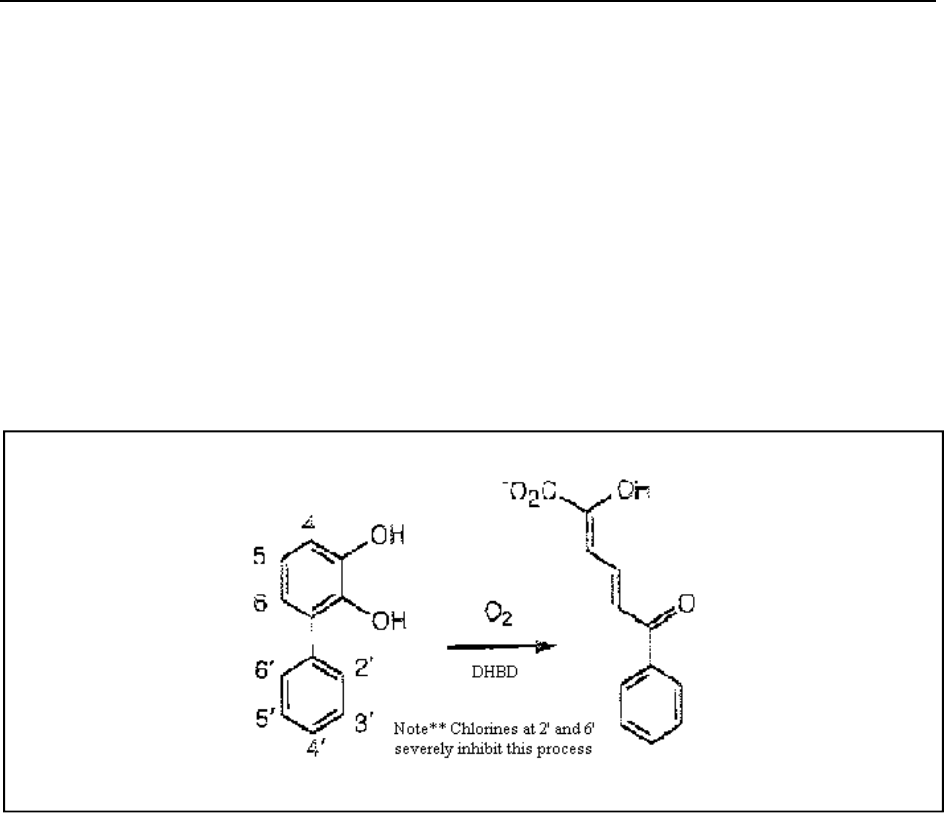
A key bottleneck has been identified in the third step of the bph pathway (Dai et al., 2002). The
third-step enzyme responsible for aromatic ring cleavage is 2,3-dihydroxybiphenyl 1,2-
dioxygenase (DHBD) (See figure 4). Dai et al. conclusively demonstrated that ortho chlorinated
Figure 4. DHBD Cleavage
(Dai et al., 2002)
2,3-dihydroxybiphenyls bind to and inhibit DHBD, promoting the enzyme’s suicide inactivation
(Dai et al., 2002). This is problematic because chlorinated 2,3-dihydroxybiphenyls are PCB
degradation products of the bph pathway. 2',6'-Dichloro-2,3-dihydroxybiphenyl has the greatest
affinity towards DHBD and causes suicide inactivation through the oxidation of active site Fe(II)
(Dai et al., 2002). As a result, ring cleavage of many ortho substituted congeners is extremely
difficult. Even the voracious PCB degrader Burkholderia LB400 is severely limited in its ability
to degrade doubly ortho substituted congeners. LB400 transforms less than 5 percent of 2,6-
dichlorbiphenyl to its analogous chlorobenzoic acid (Dai et al., 2002). Also discouraging is a new
study proving that dihydrodiols are significantly toxic to aerobic bacteria (Cámara et al., 2004). As
dihydrodiols are the products of the first step in the biphenyl pathway, there is no feasible way to
11

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
circumvent this problem. Finding a way to mitigate this toxicity might enable more rapid and
complete PCB destruction (Cámara et al., 2004).
A Superior Recombinant Strain: LB400(pR041)
The potential for aerobic bioremediation of PCBs is greatly increased by the recent development
of a superior LB400 strain (Tiedje, 2001). Researchers at Michigan State have successfully
engineered LB400(pR041) to carry the ohbRABC operon for degradation of ortho substituted
chlorobenzoates (Denef et al., 2003). The ohb genes were derived from Pseudomonas aeruginosa
142, which can degrade 2-CBA and 2,4-diCBA (Tsoi et al., 1999). LB400(pR041) effectively
grows on and mineralizes many ortho substituted PCBs (Tiedje, 2001). As a result, the strain can
be used to completely break down the majority of congeners evolved by anaerobic reductive
dechlorination (Tiedje, 2004). The mineralizing activity of LB400(pRO41) also prevents the
buildup of potentially toxic dechlorinated metabolites (Tiedje, 2001). The genome of LB400 has
been successfully closed by the Michigan State team, providing for a further understanding of its
PCB metabolism (Tiedje, 2004). The recombined LB400 strain is very stable in non-sterile soil,
and is easily the most promising organism for use in the aerobic stage of the anaerobic/aerobic
bioremediation sequence (Tiedje, 2004).
Remaining Barriers and Possible Remedies
The shortcomings of LB400(pRO41) highlight the current limitations of aerobic bioremediation
of PCBs. Most notably, LB400(pRO41) is unable to degrade doubly ortho substituted congeners
(Tiedje, 2004). PCBs with chlorines in the 2,2'- or 2,6- positions are recalcitrant to the recombined
strain, remaining extremely problematic (Tiedje, 2004). The impaired degradation of these
congeners is potentially caused by the aforementioned suicide inactivation of DHBD by ortho
substituted dihydroxybiphenyls. To improve aerobic degradation of ortho substituted congeners
like 2,6-dichlorobiphenyl, Dai et. al propose that directed evolution of DHBD could enable it to
more effectively cleave 2',6'-dichloro-dihydroxybiphenyl. Another potential solution is to lower
the binding affinity of DHBD to 2',6'-dichloro-dihydroxybiphenyl (Dai et al., 2002).
LB400(pRO41) is also susceptible to HOPDA inhibition, which prevents the efficient degradation
of many congeners (Tiedje, 2004; Bedard, 2003). The degradation of PCBs with chlorines on both
biphenyls is especially sensitive to HOPDA inhibition as the metabolic formation of 3- and 4-
chloroHOPDAs is more likely (Sylvestre, 2004).
An encouraging study published in 2004 reports that significant differences exist between the
HOPDA hydrolases of homologous organisms LB400 and R. globerulus P6 (Sylvestre, 2004). The
two hydrolases have varying HOPDA affinities and thus are inhibited by different chloroHOPDAs
(Sylvestre, 2004). An in-depth understanding of the mechanisms involved may assist the
engineering of a more capable HOPDA hydrolase (Sylvestre, 2004). To further increase the scope
of PCB congeners degraded aerobically, additional CBA pathways must be identified and
recombined into a PCB degrader. 2,4-CBA, 2,5-CBA, and 2,6-CBA would be most advantageous
12

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
(Bedard, 2003). Such pathways might enable organisms like LB400 to mineralize the enigmatic
doubly ortho substituted PCBs.
Revisiting the 1991 GE Hudson River Field Study
The last major field-scale aerobic bioremediation attempt was conducted by General Electric (GE)
in 1991. GE drove several caissons into Upper Hudson River sediments in attempt to stimulate in
situ aerobic biodegradation (Harkness et al., 1993). Nutrient supplements were provided, as well
as hydrogen peroxide as a source of dissolved oxygen (Bedard, 2003). The sediments in the
cassions were stirred to establish at least a minimal degree of mixing (Bedard, 2003). The amount
of PCB destruction was hard to measure, but there was nowhere near complete conversion of
PCBs to corresponding CBAs (Harkness et al., 1993). The major problem was once again the
limited bioavailability of the PCBs. The most recalcitrant PCBs were strongly sorbed within the
polymeric organic sediment matrix, through which PCBs must diffuse prior to desorption
(Harkness et al., 1993). Inoculation of one caisson with H850 proved ineffective, and any PCB
degradation was attributed to indigenous populations (Harkness et al., 1993), which was
surprising because the H850 was isolated from sediments in the Upper Hudson (Bedard, 2003).
As with anaerobic reductive dechlorination, bioaugmentation with indigenous organisms for
aerobic bioremediation did not seem to work.
It would be very interesting to conduct a similar test inoculating an aerated, well-mixed caisson
with LB400(pRO41) and/or RHA(pRHD34). This approach may have worked well in the Upper
Hudson, as a large buildup of 2-CBA was noted in the GE caissons (Harkness et al., 1993).
LB400(pR041) carries the ohb pathway and thus would have mineralized any evolved 2-CBA
(Tsoi et al., 1999). For such a remedial scheme to be successful, PCB bioavailability must be
improved. Another difficult task is maintaining sufficient oxygen concentrations for the aerobic
organisms (Bedard, 2003). A high degree of mixing is necessary to thoroughly remediate buried
contaminated sediments. Such stirring must be controlled to avoid scouring PCBs into the water
column. Extensive field-scale research is imperative to elucidate the many factors complicating
the in situ aerobic bioremediation of PCB-contaminated sediments.
VII. Reductive Dechlorination by Nanoscale Zero-Valent Iron
Background
Nanotechnology is rapidly expanding the limits of current remediation technologies. Nano-sized
particles have diameters between 10
-9
and 10
-7
meters and are characterized by crystalline shapes
and lattice structures (Masciangioli and Zhang, 2003). Nanoscale zero-valent iron particles have
been shown to reduce a wide range of environmental pollutants like halogenated chlorinated
solvents (Masciangioli and Zhang, 2003). Nanoscale metals have high surface area-to-volume
ratios, high surface energies, and a large fraction of stepped surface (Wang and Zhang, 1997).
Such properties combine with a unique structure and zero valency to make nano-sized metals
extremely chemically reactive (Masciangioli and Zhang, 2003). Current research is exploring the
13

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
ability of these particles to reductively dechlorinate PCBs. Chemical reduction of highly
chlorinated PCBs is greatly preferable to chemical oxidation, which can produce toxic dioxin
precursors like chlorophenols and chlorocatechols (Jackman et al., 1999).
Demonstrating the Potential of Nanoscale ZVI
Reductive dechlorination of PCBs by nanoscale zero-valent iron (ZVI) was first reported by
Zhang and Wang (Wang and Zhang, 1997). Nanoscale ZVI was synthesized through the drop-
wise addition of 1.6 M NaBH
4
to 1.0 M FeCl
3
C6H
2
0, which reduces Fe(III) to Fe(0) (Wang and
Zhang, 1997). Researchers plated nanoscale zero-valent palladium (Pd) on some of the nano-ZVI
to assess its potential as a dechlorination catalyst (Wang and Zhang, 1997). Palladized ZVI was
previously shown to completely dechlorinate Aroclors 1254 and 1260 in a short amount of time
(Grittini et al., 1995). Nano-Pd/Fe(0), regular nano-Fe(0), and commerical ZVI powder were
compared for their abilities to dechlorinate aqueous (5 mg/L PCB) Aroclor 1254 at an ambient
temperature in an ethanol/water solution (Wang and Zhang, 1997). Ethanol serves as a solvent for
PCBs and the Pd coating. Fe and Pd/Fe were added to an initial concentration of 5 g metal/100
mL solution and left in solution for 17 hours (Wang and Zhang, 1997).
No dechlorination was detected in samples amended with commercial ZVI powder having a
specific surface area of 0.9 m
2
/g (Wang and Zhang, 1997). Nanoscale ZVI (BET-specific surface
area = 33.5 m
2
/g) performed better, but still degraded at most only 25 percent of the PCBs initially
present. More encouraging was the accumulation of biphenyl in the sample, proving that certain
congeners were completely dechlorinated by the ZVI (Wang and Zhang, 1997). The higher
specific surface area of the nanoparticles increased contact between the iron and the PCBs,
facilitating reduction. The most impressive result was that the zero-valent nanoscale Pd/Fe
complex completely dechlorinated Aroclor 1254. After 17 hours, biphenyl was the only detectable
dechlorination product (Wang and Zhang, 1997).
Palladium coatings catalyze PCB dechlorination in the presence of a solvent by releasing
hydrogen previously absorbed from the surface of the iron (Korte, 2000). Upon release, the
hydrogen displaces chloride on the PCB molecule (Korte, 2000). The Pd coating increases ZVI
longevity by preventing the formation of iron oxides (Wang and Zhang, 1997). Nanoscale Pd/Fe
complexes have immense potential for the total dechlorination of PCBs, but unfortunately the
palladium coating adds a substantial production cost (Lowry, 2004). The experiment did not
investigate congener-specific dechlorination patterns or the effectiveness of nano-ZVI over an
extended period of time.
Analysis of ZVI Positional Preferences
Yak examined the dechlorination of PCBs by ZVI in subcritical water (Yak et al., 1999).
Subcritical water, characterized by extremely high temperatures and pressures, acts as a solvent to
allow significant dechlorination of Aroclor 1260 by commercial ZVI powder (Yak et al., 1999).
The ZVI converted all higher chlorinated PCBs to more lightly chlorinated congeners in a step-
14

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
wise fashion. PCBs with lower chlorine contents were more recalcitrant to reduction, but still
evolved to biphenyl (Yak et al., 1999). A subsequent study by the same researchers examined the
position-specific reductive dechlorination of PCBs in subcritical water (Yak et al., 2000).
Interestingly, the patterns were very similar to those of anaerobic microbial dechlorination. The
ZVI preferentially dechlorinates para chlorines followed by meta chlorines. As with microbial
processes, ortho chlorines are the most recalcitrant to reductive dechlorination by ZVI (Yak et al.,
2000). It is hypothesized that the ortho substituted PCBs are more resistant because their non-
coplanar orientation prevents free spinning along the C-1 carbon. This causes the electron cloud of
an ortho chlorine to hover over the opposite phenyl ring, effectively preventing reduction (Yak et
al., 2000). The results of this study prove that even ZVI has problems dechlorinating ortho
substituted congeners.
PCB Dechlorination by Micro- and Nanoscale ZVI in Contaminated Sediments
A remarkable study conducted by Dr. Kevin Gardner at the University of New Hampshire
demonstrates that ZVI can rapidly and extensively dechlorinate PCBs in contaminated sediments
(Gardner, 2004). Gardner injected microscale ZVI into PCB-laden sediments (Fe mass = 3%
sediment mass) from New Bedford Harbor and the Housatonic River (Gardner, 2002). The
preliminary results were phenomenal. The ZVI removed an estimated 84 percent of PCBs from
the Housatonic River sediments in a single day. New Bedford Harbor sediments showed more
modest results with an estimated 56 percent removal over the same time period. Substantial
biphenyl production was observed, indicating complete dechlorination of PCBs (Gardner et al.,
2004). The variance between the two sediments can be attributed to differing PCB availabilites.
Housatonic River sediments are loose and sandy and therefore do not strongly sorb to PCBs. New
Bedford sediments contain more clay and possess a “slow” desorption fraction. PCBs in the
Housatonic are thus more susceptible to attack by ZVI, as it is thought that PCBs must be in the
aqueous phase to be reduced (Gardner, 2004). Another interesting result of the experiments was
that the ZVI dechlorinated ortho chlorines almost as well as meta and para substituted chlorines
(Gardner et al., 2004). The finding of extensive ortho dechlorination by ZVI contradicts previous
studies, but if valid constitutes a major breakthrough.
The results of the UNH experiment are encouraging, but mysterious. Such efficient reduction by
microscale ZVI was unheard of, and even nanoscale ZVI (with a much greater BET-specific
surface area) degrades less than 25 percent of PCBs in a water/ethanol solution (Wang and Zhang,
1997). The UNH researchers also performed the same experiment with nanoscale ZVI and
achieved very similar results (Gardner, 2004). Confidence in the laboratory data is shaken by the
inability of Dr. Gardner to close the PCB mass balances. Biphenyl production was observed, but
the amount of biphenyl present did not correspond to the amount of PCB removed (Gardner,
2004). While the PCB removal efficiencies of Dr. Gardner’s tests are superb, his method will not
be generally accepted until he is able to account for all of the PCBs initially present in the
samples. As a result, Dr. Gardner has gone “back to the drawing board” (Gardner, 2004). Dr.
Gardner and New Hampshire researchers are currently working on the reductive dechlorination of
15

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
sediment-bound PCBs by zero-valent palladized magnesium in the presence of an ethanol
surfactant (Gardner, 2004). Results from these experiments are pending (Gardner, 2004).
Conflicting Research
Recent laboratory work by Dr. Gregory Lowry and fellow Carnegie Mellon researchers casts
doubt on the success of Dr. Gardner’s sediment experiments. The CMU team developed an
“active” sediment cap to degrade or sequester contaminants as they slowly desorb from underlying
sediments (Lowry, 2004). The incorporation of ZVI in the cap should dechlorinate desorbing
PCBs. To assess this hypothesis, Dr. Lowry tested the aqueous PCB dechlorinating ability of
micro- and nanosized ZVI at ambient conditions (Lowry, 2004). Dr. Lowry found that microscale
ZVI did not react with PCBs in a 45-day test period (Lowry et al., 2004). Nanosized ZVI
dechlorinated PCBs with congener half-lives ranging from 40 days to 77 years (Lowry et al.,
2004). No biphenyl production was noted (Lowry et al., 2004). These results vary dramatically
from the near-complete dechlorination with microscale iron observed by Dr. Gardner in a single
day. Furthermore, Lowry reports that the nano-ZVI exhibited a significant dechlorination
preference of para and meta chlorines over ortho chlorines (Lowry et al., 2004). Nanoscale ZVI
was not used in the active cap due to the noncompetitive cost of iron at the time (Lowry et al.,
2004).
Improving ZVI Longevity
Aside from its high cost, the short reactive life span of nanoscale ZVI impedes its field
applicability (Lowry, 2004). For remediation of the strongly sorbed PCBs, ZVI must remain active
in sediments and soils for many years. Ideally, an active sediment cap has a design life of
hundreds of years (Lowry, 2004). Nanoscale ZVI is so unstable and prone to oxidation that such
longevity is not feasible. Coating ZVI with palladium substantially increases the reactive life span
as previously described. Current research explores how different methods of Pd incorporation
affect ZVI deactivation rates. Traditional construction of the Pd/Fe complex plates Pd on acid-
washed base materials (Pd-Fe-A) (Gui and Gillham, 2002). A new alternative method coats
palladium on unwashed oxide-covered iron particles (Pd-Fe-U). Researchers assessed the
reactivity and longevity of Pd-Fe-A and Pd-Fe-U by using the metals to reductively dechlorinate
TCE (Gui and Gillham, 2002). Both complexes rapidly degraded TCE initially, but Pd-Fe-A
completely deactivated within 7 days, while Pd-Fe-U remained reactive throughout the 200-day
experiment (Gui and Gillham, 2002). This suggests that unwashed oxide-covered Pd plating can
significantly increase the life span of ZVI. Research has yet to explore this technology on the
nanoscale.
Synergistic Dechlorination by ZVI and Anaerobic Organisms
A novel idea is that the presence of nanoscale ZVI in soils or sediments could stimulate anaerobic
microbial reductive dechlorination. Nanoscale ZVI instantaneously drives down the oxidation-
reduction potential (ORP) of sediments upon application (Gardner, 2004). In his research, Dr.
Gardner noted sediment ORPs below -600 mV (Gardner et al., 2004). Such an environment is
16

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
immensely favorable to an assortment of anaerobic organisms like sulfate reducers and
methanogens. Furthermore, sulfate reducers and methanogens have been shown to use reducing
equivalents resulting from iron corrosion (Rosenthal et al., 2004). A zero-valent metal with a slow
deactivation rate could be used to simply drive down the ORP of contaminated sediments to incite
reductive dechlorination by indigenous or augmented PCB-dechlorinating cultures.
A recent study conclusively proves that ZVI and Dehalococcoides spp. cooperatively dechlorinate
tetrachloroethene (PCE) (Rosenthal et al., 2004). In the presence of ZVI, a mixture of two
Dehalococcoides strains completely dechlorinated PCE to ethene within 30 days (Rosenthal et al.,
2004). The two processes worked much better in conjunction than as independent reducing agents
(Rosenthal et al., 2004). The ZVI promoted favorable redox conditions and served as the electron
donor for reductive dechlorination by Dehalococcoides spp. The anaerobic corrosion of ZVI
releases hydrogen at a slow rate, selecting for dechlorinating populations over methanogens
(Rosenthal et al., 2004). This phenomenon might explain the extensive dechlorination
demonstrated by Dr. Gardner’s preliminary microscale ZVI research. The UNH project is the only
study of ZVI-induced PCB reduction in contaminated sediments, and it has yielded by far the
most encouraging results. Both the Housatonic River and New Bedford Harbor sediments are
known to contain a plethora of dechlorinating cultures (Bedard, 2003). These organisms could
have taken advantage of the low ORP and used ZVI as an electron donor ro rapidly reduce PCBs.
Enantiomeric and Isotopic Fractionation
An examination of enantiomeric and/or isotopic fractionation during synergistic dechlorination
could distinguish biotic from abiotic processes (Abraham et al., 2002). Only biological processes
can alter the enantiomeric properties of chiral compounds (Abraham et al., 2002). Pakdeesusuk et
al. have proven that the enzymatic dechlorination of certain chiral PCBs is enantioselective
(Pakdeesusuk, 2002). Evidence of enantionomeric fractionation therefore can be used to identify
microbial PCB dechlorination processes (Abraham et al., 2002). Changes in the isotopic ratios of
carbon (
13
C/
12
C) and chlorine (
37
Cl/
35
Cl) can indicate dechlorination mechanisms as well. The
microbial dechlorination of 2,3,4,5-tetrachlorobiphenyl does not enrich the heavier
13
C isotope,
signifying an absence of isotopic fractionation (Drenzek et al., 2001). If other congeners exhibit
this behavior, the depletion of
13
C levels relative to manufactured values proves biotic PCB
dechlorination to be prevalent (Drenzek et al., 2001). This conclusion can only be made if changes
in isotopic ratios are consistent with those caused by microbial dechlorination and discernable
from those caused by abiotic processes. The ubiquity of chlorine isotopic fractionation is in
question. Sediments at the New Bedford Superfund Site show a significant buildup of
37
Cl,
indicating microbial preference toward the lighter
35
Cl isotope (Abraham et al., 2002). Laboratory
work demonstrates that the microbial dechlorination of 2,3,4,5-tetrachlorobiphenyl causes no
pronounced chlorine isotopic fractionation (Drenzek et al., 2004). This report is suspect because it
is highly unlikely that the widespread dechlorination in New Bedford Harbor is attributable solely
to abiotic processes. Concrete ways of differentiating microbial and chemical dechlorination could
assist the development of nanoscale ZVI for PCB remediation.
17

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Technology Assessment
Nano- and potentially microscale zero-valent metals have great potential for in situ PCB
remediation. ZVI oxidizes to the environmentally friendly Fe(III) and can be applied through
direct subsurface injection (Gardner, 2004). Questions remain as to the effectiveness of pure nano-
ZVI, but palladium coatings can catalyze dechlorination and increase ZVI longevity. Researchers
should examine the potential uses of Fe(II) and Fe(III), the major results of ZVI oxidation. Fe(II)
might be able to reduce PCB in its own right. Native iron-reducing bacteria could turn the evolved
Fe(III) back to Fe(II) and make for a sustainable remedial cycle. The extreme oxidation state of
PCB makes this idea somewhat unreasonable, though the process has been legitimately proposed
for chlorinated ethenes (Wrenn, 2004). A more realistic idea is to promote iron-reducing cultures
that may cometabolically dechlorinate PCBs. PCB dechlorination has been shown to occur under
iron(III) reducing conditions (Wiegel and Wu, 2000). A total understanding of the fate and
transport of nanoscale ZVI is necessary prior to its commercial use in soils and sediments. Mass
balances and PCB-dechlorinating pathways must be confirmed, and the relationship between
nanoscale ZVI and dechlorinating organisms must be studied. Yet paramount to the future of ZVI
is a decreased cost of iron and palladium and an improved availability of PCBs in soils and
sediments.
VIII. The “Availability” Problem
The barrier common to all of the described in situ remediation technologies is the limited
availability of PCBs in soils and sediments. The hydrophobic nature of PCBs allows them to
tightly adsorb to organic matrices within soils and sediments, rendering them resistant to
microbial attack and chemical reduction (Mondello, 2002). There is generally a fraction of
sediment-bound PCBs that readily desorbs, as well as a “slow” fraction of strongly sorbed
particles (Gardner et al., 2004). The degree of sorption is dependent on the organic content of the
sediment/soil (Mondello, 2002). This effect is best demonstrated by Dr. Gardner’s results showing
the impairment of PCB dechlorination in the clayey, organic-rich sediments of New Bedford
Harbor (Gardner et al., 2004). Another study asserts that at most 60 percent of PCBs at any
sediment depth are available to biological or chemical processes (Mondello, 2002). Any
successful in situ remediation scheme will address the problem of PCB availability.
The most common way to increase PCB desorption is through the addition of a surfactant.
Surfactants are surface acting agents that increase solubility by lowering the interfacial surface
tension between aqueous and non-aqueous phase fluids (Abraham et al., 2002). Past experiments
using surfactant amendments to increase PCB degradation have had mixed results (Mondello,
2002). Humic substances and most other surfactants have been found to increase PCB degradation
and dechlorination yields (Fava and Piccolo, 2002), yet some surfactants adversely affect
bioremediation by decreasing microbial populations (Abraham et al., 2002). Recent surfactant
studies are very encouraging. Enzymatically synthesized maltotriose esters were shown to
substantially increase the bioavailability of Aroclor 1242 (Ferrer et al., 2003). When incubated
with LB400, Aroclor 1242-contaminated soil amended with such a surfactant showed a 92 percent
18

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
decrease in Aroclor concentration (Ferrer et al., 2003). PCB solubility was increased from 140 to
305
:g/L (Ferrer et al., 2003). Most importantly, the high degradation rates prove the surfactant
was non-toxic to LB400.
It has been reported that randomly methylated-beta-cyclodextrin (RAMEB) substantially increases
PCB bioavailability while simultaneously stimulating PCB-degrading aerobic bacteria (Fava et al.,
2003b). Researchers treated PCB-contaminated soil with varying amounts of RAMEB in small
reactors. RAMEB greatly increased the fraction of aqueous PCBs and slowly degraded in the
presence of indigenous soil organisms (Fava et al., 2003b), which was very beneficial as the
natural degradation of the surfactant actually promoted biphenyl- and chlorobenzoate-degrading
populations. In recent work, Dr. Gardner uses ethanol to extract PCBs from sediment matrices
(Gardner, 2004). Ethanol is advantageous because it is cheap and environmentally friendly
(Gardner, 2004). Ethanol also acts as a solvent to release hydrogen from the Pd/Fe complex,
catalyzing dechlorination. Results from Dr. Gardner’s work are pending.
Electrokinetic manipulation of sorbed PCBs also can increase the contaminant’s availability to
microbial or chemical processes. Oxford University researchers are currently exploring the ability
of electric currents to desorb PCBs and move them micrometers in soil (Jackman, 2004). Control
of the induced PCB movement is essential to ensure the contaminant’s bioavailability to
organisms in the soil (Jackman, 2004). While some sediments or soils are more conducive to
desorption, surfactants or electrodes must be used in any in situ PCB remediation scheme. The
“slow” PCB fraction otherwise will persist. An efficient and environmentally friendly method of
PCB manipulation must be uncovered to allow in situ eradication of the contaminant.
IX. Conclusion
Despite years of research and many promising leads, an effective in situ remediation technique for
PCB-contaminated soils and sediments does not exist. A sequential anaerobic/aerobic bioremedia-
tion system has always exhibited enormous potential at the laboratory scale. Dechlorinating
cultures o-17 and DF-1 have been identified, and research is on the verge of isolating PCB-
respiring organisms (Wu et al., 2002). Ways to prime dechlorination are established, and
dechlorination pathways are well known (Bedard, 2003). Aerobic strains LB400(pRO41) and
RHA(pRHD34) adeptly grow on and mineralize most of the major anaerobic dechlorination
products (Tiedje, 2004). Doubly ortho substituted congeners remain recalcitrant to the recombined
strain, but genetic engineering can circumvent the problem by preventing DHBD inhibition (Dai
et al., 2002). Comprehensive field-scale research must be conducted to advance bioremediation
technology.
Nanosized ZVI is a proven PCB dechlorinator that works swiftly and efficiently. Most inspiring is
the notion of a chemical reduction/biological oxidation sequence for complete mineralization of
PCBs. Nanoscale ZVI, especially when palladized, is a voracious dechlorinator and can rapidly
reduce Aroclor mixtures to congeners susceptible to aerobic degradation. The use of an
19

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
environmentally benign surfactant could greatly augment the removal efficiencies of such
processes. Alternatively, “active” sediment caps can be used to dechlorinate PCBs as they desorb
from sediment matrices. Such sediment caps must maintain reactivity for extended durations.
Pilot- or field-scale tests of these technologies are needed to further assess their strengths and
shortcomings.
A controversial barrier to the in situ remediation of PCBs yet to be addressed in this paper is the
general public phobia of genetically modified organisms (GMOs) and nanotechnology. While
many fears are unjustified, the use of GMOs and nanomaterials must be strictly monitored as
several legitimate concerns do exist. Control mechanisms must prevent the environmental
dispersion of engineered genes (Sylvestre, 2004). Active-and-passive biological containment
(ABC) systems are being developed that trigger a “killing” gene in response to an environmental
signal (Sylvestre, 2004). Mastery of ABC techniques might quell the public distrust of GMOs.
Nanoparticles are feared to enter the food chain, self-replicate, and facilitate the dissemination of
non-targeted pollutants (Masciangioli and Zhang, 2003). A better understanding of the behavior of
nanomaterials in sediments and soils is necessary. Control techniques for GMOs and nano-
materials are important, but public hysteria should not hinder the advancement of the most
promising agents for the in situ remediation of PCB-contaminated soils and sediments.
X. Citations
Abraham, Wolf Ranier, Balbina Nogales, Peter N. Golyshin, Dietmar H. Pieper, and Kenneth N.
Timmis. 2002. “Polychlorinated Biphenyl-Degrading Communities in Soils and Sediments.”
Current Opinion in Microbiology 5(3):246-253.
Baars, A.J., M.I. Bakkera, R.A. Baumanna, P.E. Boonb, J.I. Freijera, L.A.P. Hoogenboomb, R.
Hoogerbruggea, J.D. van Klaverenb, A.K.D. Liema, W.A. Traagb, and J. de Vriesc. 2004.
“Dioxins, Dioxin-Like PCBs and Non-Dioxin-Like PCBs in Foodstuffs: Occurrence and
Dietary Intake in The Netherlands.” Toxicology Letters 151(1):51-61.
Bedard, Donna L. and Ralph J. May. 1996. “Characterization of the Polychlorinated Biphenyls in
the Sediments of Woods Pond: Evidence for Microbial Dechlorination of Aroclor 1260 In
Situ.” Environmental Science and Technology 30(1):237-245.
Bedard, Donna L. 2003. “Polychlorinated Biphenyls in Aquatic Sediments: Environmental Fate
and Outlook for Biological Treatment.” Dehalogenation: Microbial Processes and
Environmental Applications, M.M. Haggblom and I. Bossert, eds., Kluwer Press:443-465.
Berkaw, Mary, Kevin R. Sowers, and Harold D. May. 1996. “Anaerobic ortho Dechlorination of
Polychlorinated Biphenyls by Estuarine Sediments from Baltimore Harbor.” Applied and
Environmental Microbiology 62(7):2534-2539.
20

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Cámara, Beatriz, Cristina Herrera, Myriam González, Eduardo Couve, Bernd Hofer, and Michael
Seeger. 2004. “From PCBs to Highly Toxic Metabolites by the Biphenyl Pathway.”
Environmental Microbiology 6(8):842-850.
Cho, Young-Cheol, Roger C. Sokol, Robert C. Frohnhoefer, and G-Yull Rhee. 2003. “Reductive
Dechlorination of Polychlorinated Biphenyls: Threshold Concentration and Dechlorination
Kinetics of Individual Congeners in Aroclor 1248.” Environmental Science and Technology
37(24):5651-5656.
Cutter, L., J. Watts, K. Sowers, and H. May. 2001. “Identification of a Microorganism that Links
its Growth to the Reductive Dechlorination of 2,3,5,6-Chlorobiphenyl.” Environmental
Microbiology 3(11):699-709.
Dai, Shaodong, Frédéric H. Vaillancourt, Halim Maaroufi, Nathalie M. Drouin, David B. Neau,
Victor Snieckus, Jeffrey T. Bolin, and Lindsay D. Eltis. 2002. “Identification and Analysis of
a Bottleneck in PCB Biodegradation.” Nature Structural Biology 9(12):934-939.
Denef, Vincent J., Joonhong Park, Jorge L.M. Rodrigues, Tamara V. Tsoi, Syed A. Hashsham,
and James M. Tiedje. 2003. “Validation of a More Sensitive Method for Using Spotted
Oligonucleotide DNA Microarrays for Functional Genomic Studies on Bacterial
Communities.” Environmental Microbiology 5(10):933-943.
Drenzek, Nicholas J., Timothy I. Eglinton, Carl O. Wirsen, Harold D. May, Qingzhong Wu,
Kevin R. Sowers, and Christopher M. Reddy. 2001. “The Absence and Application of Stable
Carbon Isotopic Fractionation during the Reductive Dechlorination of Polychlorinated
Biphenyls.” Environmental Science and Technology 35(16):3310-3313.
Drenzek, Nicholas J., Timothy I. Eglintona, Carl O. Wirsenb, Neil C. Sturchioc, Linnea J.
Heratyc, Kevin R. Sowersd, Qingzhong Wue, Harold D. Maye, and Christopher M. Reddy.
2004. “Invariant Chlorine Isotopic Signatures During Microbial PCB Reductive
Dechlorination.” Environmental Pollution 128(3):445-448.
Faroon, O., D. Jones, and C. de Rosa. 2001. “Effects of Polychlorinated Biphenyls on the Nervous
System.” Toxicology and Industrial Health 16(7-8):305-33.
Fava, F. and A. Piccolo. 2002. “Effects of Humic Substances on the Bioavailability and Aerobic
Biodegradation of Polychlorinated Biphenyls in a Model Soil.” Biotechnology and
Bioengineering 77(2):204-11.
21

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Fava, F., G. Zanaroli, and L. Y. Young. 2003a. “Microbial Reductive Dechlorination of
Pre-Existing PCBs and Spiked 2,3,4,5,6-Pentachlorobiphenyl in Anaerobic Slurries of a
Contaminated Sediment of Venice Lagoon (Italy).” FEMS Microbiology Ecology 44(3):309-
318.
Fava F., L. Bertin, S. Fedi, and D. Zannoni. 2003b. “Methyl-beta-Cyclodextrin-Enhanced
Solubilization and Aerobic Biodegradation of Polychlorinated Biphenyls in Two
Aged-Contaminated Soils.” Biotechnology and Bioengineering 81(4):381-90.
Fennell, Donna E., Ivonne Nijenhuis, Susan F. Wilson, Stephen H. Zinder, and Max M.
Häggblom. 2004. “Dehalococcoides ethenogenes Strain 195 Reductively Dechlorinates
Diverse Chlorinated Aromatic Pollutants.” Environmental Science and Technology
38(7):2075-2081.
Ferrer M., P. Golyshin, and K.N. Timmis K.N. 2003. “Novel Maltotriose Esters Enhance
Biodegradation of Aroclor 1242 by Burkholderia cepacia LB400.” World Journal of
Microbiology and Biotechnology 19(6):637-643.
Gardner, Kevin. 2002. In-Situ Treatment of PCBs in Marine and Freshwater Sediments Using
Colloidal Zero-Valent Iron: CICEET Progress Report for the period 03/01/02 through
09/01/02. Cooperative Institute for Coastal and Estuarine Environmental Technology
(CICEET). http://ciceet.unh.edu/progressreports/2002/fall/gardner/index.html
Gardner, Kevin. 2004. New Hampshire University. Telephone interview, July 2004.
Gardner, Kevin, Deana Aulisio, and Jean M. Spear. 2004. “In-Situ Dechlorination of
Polychlorinated Biphenyls in Sediments Using Zero-Valent Iron.” PowerPoint presentation
from the RTDF Sediments meeting of February 18-19, 2004.
Grittini, Carina, Mark Malcomson, Quintus Fernando, and Nic Korte. 1995. “Rapid
Dechlorination of Polychlorinated Biphenyls on the Surface of a Pd/Fe Bimetallic System.”
Environmental Science and Technology 29(11):2898-900.
Gruden, Cyndee L., Q. Shiang Fu, Andre L. Barkovskii, Iris D. Albrecht, Mary M. Lynam, and
Peter Adriaens. 2003. “Dechlorination of Sediment Dioxins: Catalysts, Mechanisms, and
Implications for Remedial Strategies and Dioxin Cycling.” Dehalogenation: Microbial
Processes and Environmental Applications, M.M. Haggblom and I.D. Bossert, eds., Kluwer
Academic Press:347-372.
Gui, Lai and Robert W. Gillham. 2002. “Evaluating the Performance of Palladium-plated
Granular Iron for Reductive Dechlorination of TCE.” Groundwater Quality: Natural and
22

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Enhanced Restoration of Groundwater Pollution (Proceedings of the Groundwater Quality
2001 Conference held at Sheffield, UK, June 2001). IAHS Publ. no. 275:403-408.
Halden, Rolf U. and Daryl F. Dwyer. 1997. “Biodegradation of Dioxin-Related Compounds: A
Review.” Bioremediation Journal 1(1):11-25.
Harkness, M R, J.B. McDermott, D.A. Abramowicz, J.J. Salvo, W.P. Flanagan, M.L. Stephens,
F.J. Mondello, R.J. May, J.H. Lobos, K.M. Carroll, M.J. Brennan, A.A. Bracco, K.M. Fish,
G.L. Warner, P.R. Wilson, D.K. Dietrich, D.T. Lin, C.B. Morgan, and W.L. Gately. 1993. “In
Situ Stimulation of Aerobic PCB Biodegradation in Hudson River Sediments.” Science
259(5094):503-507.
Jackman, Simon. 2004. Oxford University. Telephone interview, June 2004.
Jackman, Simon A., Christopher J. Knowles, and Gary K. Robinson. 1999. “SACRED - A Novel
Catalytic Process for the Environmental Remediation of Polychlorinated Biphenyls (PCBs).”
Chemosphere 38(8):1889-1900.
Johnson, Barry L., Heraline E. Hicks, William Cibulas, Obaid Faroon, Annette E. Ashizawa,
Christopher T. De Rosa, Vincent J. Cogliano, and Milton Clark. [1998] Public Health
Implications of Exposure to Polychlorinated Biphenyls (PCBs). Agency for Toxic Substances
and Disease Registry, Atlanta, GA. www.atsdr.cdc.gov/DT/pcb007.html
Kenyon College, 2004. Burkholderia. The Microbial Biorealm Web site.
http://biology.kenyon.edu/Microbial_Biorealm/bacteria/proteobacteria/Burkholderia/Burkhold
eria.htm
Kim, Jongseol and G-Yull Rhee. 1997. “Population Dynamics of Polychlorinated
Biphenyl-Dechlorinating Microorganisms in Contaminated Sediments.” Applied and
Environmental Microbiology 63(5):1771-1776.
Korte, Nic. 2000. “Dechlorination of Polychlorinated Biphenyls (PCBs) in Soil.” Minutes of the
46th meeting of the Naval Station (NAVSTA) San Diego Restoration Advisory Board (RAB),
26 January 2000.
Lowry, Greg. 2004. Carnegie Mellon University. Telephone interview, July 2004.
Lowry, Greg, Paul J. Murphy, and Kathleen M. Johnson. 2004. “Development and In Situ
Application of Sorbent/Reagent-Amended ‘Active’ Sediment Caps for Managing HOC-
contaminated Sediments.” Abstract from the Technology Benchmarking Workshop on
Sediment and Floodplain Remediation, March 25-26, 2004, University of Michigan.
23

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Manzano, Manuel A., Jose A. Perales, Diego Sales, and Jose M. Quiroga. 2003. “Enhancement of
Aerobic Microbial Degradation of Polychlorinated Biphenyls in Soil Microcosms.”
Environmental Toxicology and Chemistry 22(4):699-705.
Masciangioli, Tina and Wei-xian Zhang. 2003. “Environmental Technologies at the Nanoscale.”
Environmental Science and Technology 37(5):102A-108A.
Mondello, F.J. 2002. Microbial Bioremediation of Polychlorinated Biphenyls: Applicability to the
Former GE Canada Transformer Manufacturing Facility Located in Guelph, Ontario.
General Electric Company. GE Global Research Technical Information Series, Report no.
2002GRC022. www.crd.ge.com/cooltechnologies/pdf/2002grc022.pdf
Natarajan, M.R., J. Nye, W.M. Wu, H. Wang, and M.K. Jain. 2000. “Reductive Dechlorination of
PCB-Contaminated Raisin River Sediments by Anaerobic Microbial Granules.”
Biotechnology and Bioengineering 55(1):182-190.
Natarajan, M.R., W.M. Wu, J. Nye, and H. Wang. 1996. “Dechlorination of Polychlorinated
Biphenyl Congeners by an Anaerobic Microbial Consortium.” Applied Microbiology and
Biotechnology 46(5-6):673-677.
Pakdeesusuk, Usarat, W. Jack Jones, Cindy M. Lee, Arthur W. Garrison, Walter L. O’Niell,
David L. Freedman, John T. Coates, and Charles S. Wong. 2003. “Changes in Enantiomeric
Fractions During Microbial Reductive Dechlorination of PCB132, PCB149, and Aroclor 1254
in Lake Hartwell Sediment Microcosms.” Environmental Science and Technology 37(6):1100-
1107.
Quantitative Environmental Analysis, LLC (QEA). 1999. PCBs in the Upper Hudson: Executive
Summary. General Electric Company.
www.ge.com/files/usa/en/commitment/ehs/hudson/Executive_Summary.pdf
Rahuman, Mujeebur, Luigi Pistone, Ferruccio Trifirò, and Stanislav Miertus. 2000. “Destruction
Technologies for Polychlorinated Biphenyls.” Proceedings of Expert Group Meetings on
POPs and Pesticides Contamination: Remediation Technologies (April 2000) and on Clean
Technologies for the Reduction and Elimination of POPs (May 2000). United Nations
Industrial Development Organization (ICS-UNIDO).
Richardson, Ruth. 2004. Cornell University. Telephone interview, June 2004.
Rodrigues, Jorge L.M., Olga V. Maltseva, Tamara V. Tsoi, Rebekah R. Helton, John F. Quensen
III, Masao Fukuda, and James M. Tiedje. 2001. “Development of a Rhodococcus
24

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Recombinant Strain for Degradation of Products from Anaerobic Dechlorination of PCBs.”
Environmental Science and Technology 35(4):663-668.
Rosenthal, Heidrun, Lorenz Adrian, and Martin Steiof. 2004. “Dechlorination of PCE in the
Presence of Fe
0
Enhanced by a Mixed Culture Containing Two Dehalococcoides Strains.”
Chemosphere 55(5):661-669.
Sylvestre, Michel. 2004. “Genetically Modified Organisms to Remediate Polychlorinated
Biphenyls. Where Do We Stand?” International Biodeterioration & Biodegradation
54(2/3):153-162.
Tiedje, James. 2004. Michigan State University. Telephone interview, June 2004.
Tiedje, James. 2001. Project 3: PCB Bioremediation Strategies and Potential Intermediates of
Toxicological Significance, 2001 Progress. National Institute of Environmental Health
Sciences, Superfund Basic Research Program: Health Hazards from Groundwater
Contamination. www-apps.niehs.nih.gov/sbrp/program2000/ResProg.cfm?ProjAuto
Num=449&Yr=2001-2002&Abbr=MSUIII&ONum=336&outreach=’‘
Tsoi, Tamara V., Elena G. Plotnikova, James R. Cole, William F. Guerin, Michael Bagdasarian,
and James M. Tiedje. 1999. “Cloning, Expression, and Nucleotide Sequence of the
Pseudomonas aeruginosa 142 ohb Genes Coding for Oxygenolytic ortho Dehalogenation of
Halobenzoates.” Applied and Environmental Microbiology 65(5):2151-2162.
U.S. Environmental Protection Agency (U.S. EPA). 2003. Hudson River PCBs Superfund Site.
Office of Superfund Remediation and Technology Innovation Web site.
www.epa.gov/superfund/accomp/success/hudson.htm
U.S. Environmental Protection Agency (U.S. EPA). 1997. Management of Polychlorinated
Biphenyls in the United States. Office of Pollution Prevention and Toxics, Washington, DC.
www.chem.unep.ch/pops/indxhtms/cspcb05.html
Voie, Øyvind Albert, Arnt Johnsen, and Helle Kristin Rossland. 2002. “Why Biota Still
Accumulate High Levels of PCB After Removal of PCB Contaminated Sediments in a
Norwegian Fjord.” Chemosphere 46(9-10):1367-1372.
Wang, Chuan-Bao and Wei-xian Zhang. 1997. “Synthesizing Nanoscale Iron Particles for Rapid
and Complete Dechlorination of TCE and PCBs.” Environmental Science and Technology
31(7):2154-2156.
25

Emerging Technologies for the In Situ Remediation of PCB-Contaminated Soils and Sediments:
Bioremediation and Nanoscale Zero-Valent Iron
Wiegel, Juergen and Qingzhong Wu. 2000. “Microbial Reductive Dehalogenation of
Polychlorinated Biphenyls.” FEMS Microbial Ecology 32(1):1-15.
Wrenn, Brian A. 2004. “Enhanced Reductive Dechlorination through Biological Interactions with
Zero-Valent Iron.” Abstract from the Federal Remediation Technologies Roundtable General
Meeting of June 9, 2004.
Wu, Qingzhong, Joy E. M. Watts, Kevin R. Sowers, and Harold D. May. 2002. “Identification of
a Bacterium That Specifically Catalyzes the Reductive Dechlorination of Polychlorinated
Biphenyls with Doubly Flanked Chlorines.” Applied and Environmental Microbiology
68(2):807-812.
Yak, Hwa K., Bernd W. Wenclawiak, I. Francis Cheng, John G. Doyle, and Chien M. Wai. 1999.
“Reductive Dechlorination of Polychlorinated Biphenyls by Zerovalent Iron in Subcritical
Water.” Environmental Science and Technology 33(8):1307-1310.
Yak, Hwa K., Qingyong Lang, and Chien M. Wai. 2000. “Relative Resistance of Positional
Isomers of Polychlorinated Biphenyls toward Reductive Dechlorination by Zerovalent Iron in
Subcritical Water.” Environmental Science and Technology 34(13):2792-2798.
Zwiernik, Matthew J., John F. Quensen III, and Stephen A. Boyd. 1998. “FeSO
4
Amendments
Stimulate Extensive Anaerobic PCB Dechlorination.” Environmental Science and Technology
32(21):3360-3365.
26
